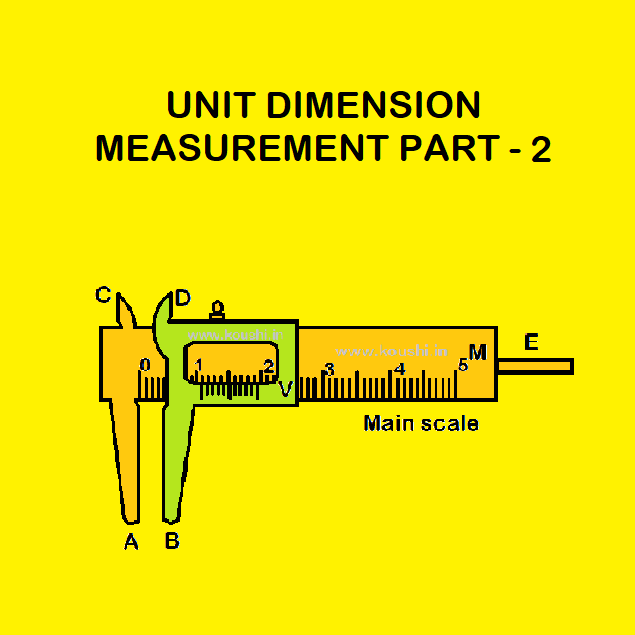
Measurement:
1. Parallax method: If you hold a pen in front of your eye and look the tip of the pen by closing the right eye and then the left eye keeping right eye open, you observe that the position of the tip of the pen changes. This relative shift of the tip of the pen with respect to background is called parallax. The distance between two points of observations (the distance between your eyes) is called the basis. The angle subtended by basis at the object is called parallactic angle.
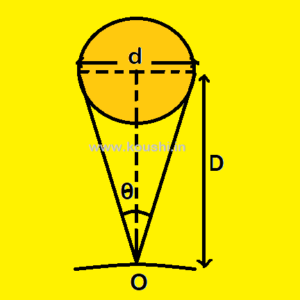
If a planet of diameter d is at a distance D (D ![]() d) and
d) and ![]() is the parallactic angle, then
is the parallactic angle, then ![]() is very small. From diagram we can write, tan
is very small. From diagram we can write, tan ![]() =
= ![]()
![]()
![]() =
= ![]() .
.
Example: The distance between a star and earth is 1.5 ![]() 1011 m. If the angular diameter of the star is 1500// then find the diameter of the star?
1011 m. If the angular diameter of the star is 1500// then find the diameter of the star?
![]() = 1500// =
= 1500// = ![]() rad
rad
Distance between a star and earth is D = 1.5 ![]() 1011 m.
1011 m.
Diameter of the star (d) = D ![]() =
= ![]() = 1.091
= 1.091 ![]() 1011 m.
1011 m.
2. Calculation of radius of atom by using Avogadro’s hypothesis:
Let us consider a 1g of substance of molecular weight M. We want to calculate the radius of an atom of that substance.
If N is the Avogadro’s number then number of atoms in 1g of substance is ![]() .
.
If r is the radius of each atom then volume of one atom is ![]() .
.
The volume of total atoms in 1g of substance is ![]() .
.
If ![]() is the density of the substance then volume of 1g of the substance is v =
is the density of the substance then volume of 1g of the substance is v = ![]()
According to Avogadro’s hypothesis the actual volume occupied by the atoms in any given mass of a substance is about two-third of the volume of that substance.
The actual volume of atom in 1g of substance = ![]() of the volume of 1g of substance
of the volume of 1g of substance
Therefore, ![]() =
= ![]()
![]() r =
r = ![]() .
.
Calculation of molecular size: We want to calculate the molecular size of oleic acid. 1 cc of oleic acid is mixed with 500cc of alcohol so that the concentration of oleic acid in this solution is ![]() cc of oleic acid per cc of alcohol. Water is taken in a beaker and some lycopodium powder is sprinkled over it. Let n drops of oleic acid solution is put on water. Lycopodium powder does not allow the drops to come in contact with water and dissolve in it. After some time alcohol evaporates leaving behind a film of oleic acid on water and the thickness of the film is compared as the size of oleic acid molecule. The area of thin layer is measured by tracing paper and graph paper.
cc of oleic acid per cc of alcohol. Water is taken in a beaker and some lycopodium powder is sprinkled over it. Let n drops of oleic acid solution is put on water. Lycopodium powder does not allow the drops to come in contact with water and dissolve in it. After some time alcohol evaporates leaving behind a film of oleic acid on water and the thickness of the film is compared as the size of oleic acid molecule. The area of thin layer is measured by tracing paper and graph paper.
If v is the volume of each drop of solution then volume of oleic acid in n drops is ![]() cc
cc
If A is the area of the film, then the thickness of the film = molecular size of oleic acid = ![]() cm.
cm.
Click the button to go to the previous part of this chapter.
Click the button to go to the next part of this chapter.